How To Check If A Company Is Fda Registered
Are In that location "FDA Registered" or "FDA Certified" Medical Devices? How Exercise I Know What Is FDA Canonical?
You may have seen words like this on a website selling a medical device in the United States, sometimes even with an FDA logo:
- FDA Registered
- FDA Certified
- FDA Registration Certificate
Such words may be used to mislead yous. Is that the aforementioned thing equally FDA approved, FDA cleared, or FDA authorized? The curt answer is NO. Here's why.
FDA Registration
Owners or operators of places of business (too chosen establishments or facilities) that are involved in the production and distribution of medical devices intended for use in the U.s.a. are generally required to annals annually with the FDA.
It's important to understand:
When a facility registers its establishment and lists its devices, the resulting entry in the FDA's registration and listing database does not denote approval, clearance, or authority of that facility or its medical devices.
For more details:
- For data on medical device establishment registration, see How to Report and Market Your Device > Device Registration and List.
- For data on FDA establishment registration in general, see FDA Basics for Industry > Registration and Listing.
Are in that location FDA Certificates?
When a business involved in the production and distribution of medical devices intended for use in the Us registers with the FDA, they do non receive a certificate from the FDA.
It'due south important to understand:
The FDA does not event any type of device registration certificates to medical device facilities.
In addition, the FDA does non "certify" registration information for businesses that accept registered and listed.
Misleading FDA Registration Certificates
Some firms sell medical devices in the Us alongside "FDA registration certificates," such as the sample certificate depicted hither.
These certificates often take the expect of an official government document and may include the FDA logo. However, FDA does not event device registration certificates.
Firms that misleadingly display certificates alongside information nearly and photos of a device for auction in the U.s.a. to imply review or approving past FDA of the device misbrand the device in violation of the Federal Food, Drug, and Cosmetic Act.
Related information: FDA Calls on Sure Firms to Stop Producing and Issuing Misleading "FDA Registration Certificates"
To report suspected misuse of a registration certificate, please refer to Reporting Allegations of Regulatory Misconduct.
How Do You Know if the FDA Approved, Cleared, or Authorized a Medical Device?
The FDA provides several ways for y'all to check if the FDA approved or cleared a medical device or, every bit described below, if the FDA authorized the device to be used during a public wellness emergency.
Check for Approved and Cleared Products in the Devices@FDA Database: Devices@FDA is a catalog of canonical and cleared medical device information from the FDA.
To search for FDA-approved or FDA-cleared products past device name or company proper noun:
- Go to the Devices@FDA Database.
- In the Enter a search term in the space beneath field, type the proper name of the device or the company name. You can type the verbal proper name of a specific device or a generic name for a category of devices (such every bit pacemaker).
- Click Search.
Example: Search for Pacemaker
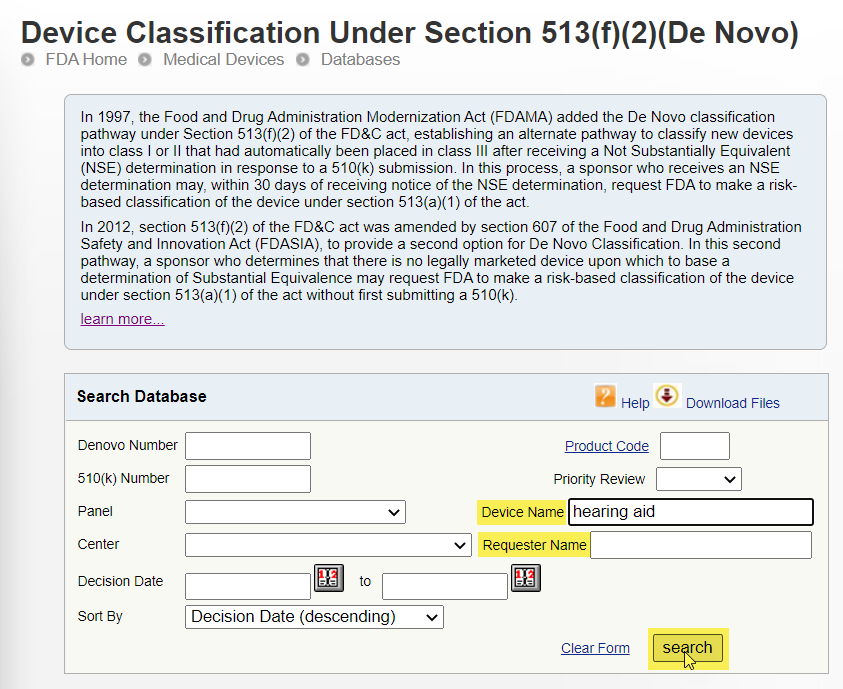
Check for Products in the De Novo Database: The FDA may review medical devices through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type. A device reviewed through this pathway may be authorized for marketing in the Us. The term "De Novo" is Latin for "new."
To search for products by device proper name or company proper name:
- Go to the Device Classification Under Section 513(f)(2)(De Novo) database.
- Practice i of the post-obit:
- In the Device Name field, type the device name and click Search.
- In the Requester Name field, type the company proper noun and click Search.
Example: Search for Hearing Aids
Check for Emergency Employ Authorizations: In certain types of public health emergencies, such as during the COVID-19 pandemic, when the Secretary of Health and Homo Services declares that circumstances exist justifying the authorization of emergency utilise of medical devices, the FDA may upshot an Emergency Use Authorization (EUA) to authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, care for, or foreclose serious or life-threatening diseases or conditions when certain criteria are met. This can assistance provide more than timely access to critical medical devices that may assist during the emergency when there are no adequate, approved, and bachelor options.
To check medical device EUAs, get to Emergency Use Authorizations for Medical Devices.
How To Check If A Company Is Fda Registered,
Source: https://www.fda.gov/medical-devices/consumers-medical-devices/are-there-fda-registered-or-fda-certified-medical-devices-how-do-i-know-what-fda-approved
Posted by: steinerthreaske1987.blogspot.com




0 Response to "How To Check If A Company Is Fda Registered"
Post a Comment